The Research Ethics Committee is Managed by Dr Sadhbh O’Neill, Sadhbh.ONeill@tuh.ie
- The Chair of the JREC is Prof Anne-Marie Tobin. Please contact researchethics@tuh.ie and queries will be forwarded to Prof Tobin.
PURPOSE OF THE JREC
- The St. James’ Hospital (SJH) / Tallaght University Hospital (TUH) Joint Research Ethics Committee (REC) provides ethical review of research studies in which patients, staff and sometimes healthy volunteers are either:
- Direct participants (Clinical Trial, Observational Study)
- Their medical records are used (Retrospective Chart Review)
- Their biological samples are used (Clinical Trial, Observational Study)
- The aim of the JREC is to ensure the safety of our participants at all times
TYPES OF STUDIES THE JREC REVIEW
- Clinical Trials:
- The SJH/TUH JREC is a Recognised REC, thus the JREC is approved to provide ethical review of clinical trials of medicinal products (CTIMP) as required under European Communities Regulation (S.I. Number 190/2004).
- The SJH/TUH can review Clinical Trials which are multi-site i.e. REC approval for a Clinical Trial is only required from one Recognised REC in Ireland for multi-site studies
- All Clinical Trials require a contract between the Principle Investigator (PI), the Hospital and the Sponsor (LINK TO CT SIGN OFF)
- PLEASE NOTE: A new European Clinical Trials Regulation (536/2014) has been adopted and will be implemented in Ireland. In anticipation of the implementation of this Regulation, the Department of Health have established the National Research Ethics Committee (NREC). In May 2021 the NREC will begin reviewing CTIMP applications. As of the 17th November the JREC will no longer review CTIMPs.
- Observational Research Studies:
- Observational Research studies involve recruitment of patients or staff to the research study. The study may include a chart review, bio-specimen procurement, interviews, questionnaires, etc.
- Retrospective Chart Review:
- Retrospective chart reviews are considered research. They do not require consent if the researcher is a Health Practitioner or other employee of the Data Controller (SJH or TUH) and would normally have access to patient charts. The study must also be low risk with respect to data protection (Please see Data Protection Section - LINK).
HOW THE SJH/TUH JREC FUNCTIONS
- The SJH/TUH REC follows the Operational Procedures for RECs Guidance issued by the Irish Council for Bioethics (2004) (available at: Irish Council for Bioethics)
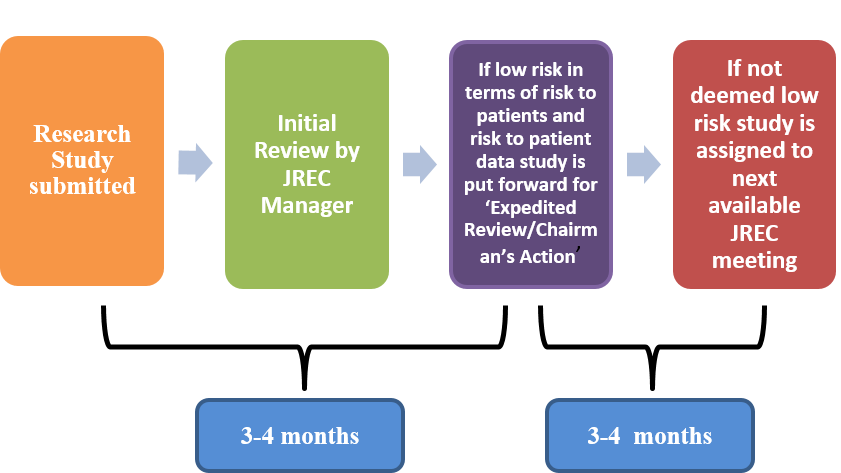
- The full REC meets 10 times each year and reviews between 6 and 10 applications at each meeting.
- Researchers must submit all research studies to the JREC for review via the online Ethics Review Manager
- As of May 2021 the JREC no longer reviews Clinical Trials, or Device Trials (subject to legislation), which are instead submitted to the National REC (NREC). Device Trials (not subject to legislation) may be submitted via the online review portal.
- If applicable, submissions are booked into the next available committee meeting (please note timelines below).
- If reviewed at the committee meeting, a letter with review comments will be issued to the applicant within 2 weeks of the committee meeting date.
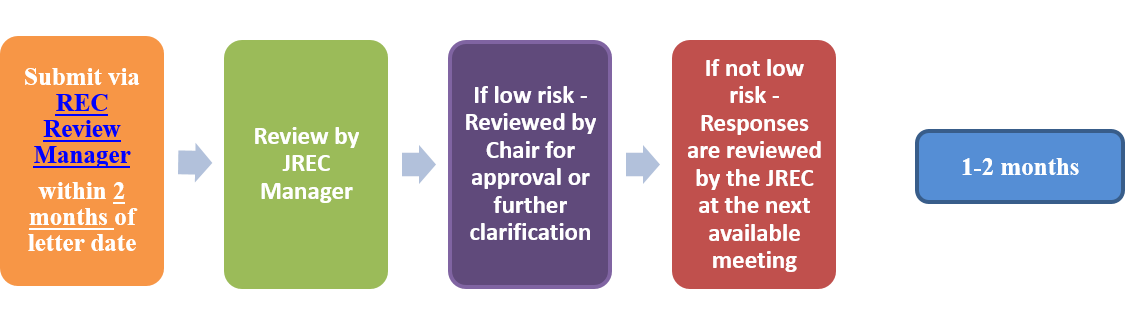
- Submissions following initial review or approval for ALL types of studies:
- Response to comments:
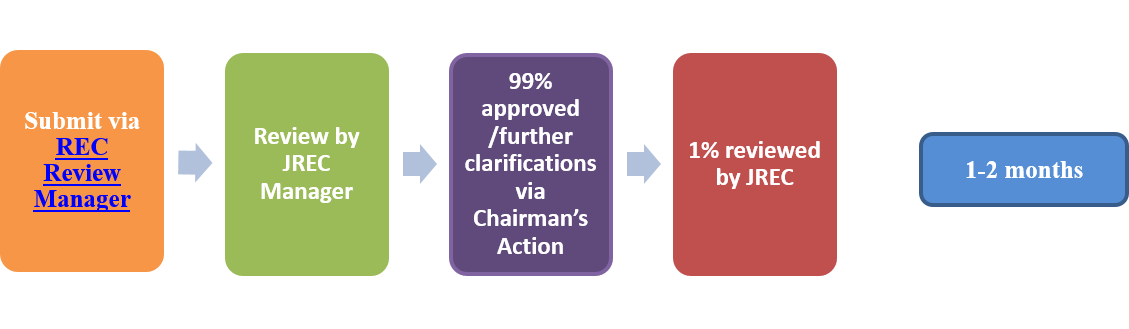
- Amendments:
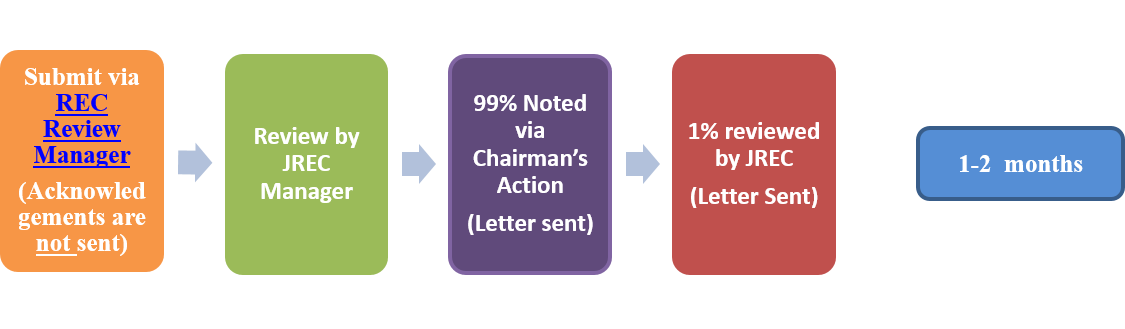
- Reports/Notifications:
- The REC issues one of the following decisions in relation to a valid application:
I. The study is APPROVED (Final Opinion): The study is approved. The applicant may conduct the research as outlined in the application form submitted to the REC.
II. The study is PROVISIONALLY APPROVED: The study is approved subject to recommended revisions to the application and/or responses to questions posed. The applicant must resubmit any revisions and/or responses to the REC before receiving final approval for the study. No research may be conducted prior to receiving final approval.
III. Further Information required: The REC requires further information from the applicant and/or referee (expert opinion) before a decision can be reached.
IV. A decision on study approval is DECLINED: The REC has not approved the study. The applicant is given a full explanation of the REC’s decision with or without an invitation to resubmit a substantially altered proposal for reconsideration.
ADDITIONAL INFORMATION
- Health Research Consent Declaration Committee (HRCDC):
- Researchers conducting studies involving patients who lack capacity to give their own informed consent or researchers who do not plan on seeking explicit consent from the patient must apply to the HRCDC for a consent declaration for the processing of the patients data. Provisional JREC approval must be in place before applying to the HRCDC.
- Health Products Regulatory Authority (HPRA):
- Research involving Investigational Medicinal Products or Devices may require approval from the HPRA. Please consult with the HPRA regarding your study before applying to the JREC.
Committee Meeting Dates 2023:
January 24th 2024
February 21st 2024
March 20th 2024
April 17th 2024
May 22nd 2024
June 19th 2024
July 24tht 2024
August – No Meeting
September 18th 2024
October 23rd 2024
November 20th 2024
December – No Meeting
TYPES OF STUDIES THE JREC DO NOT REVIEW
Clinical Audit:
- Clinical audit is the systematic review and evaluation of current practice against standards with a view to improving clinical care for service users.
Quality Improvement Initiatives:
- Improving quality is about making healthcare safe, effective, patient – centred, timely, efficient and equitable.
- Quality Improvement is the framework we use to systematically improve the ways care is delivered to our patients. Healthcare consists of thousands of interlinked processes that result in a very complex system. Processes are defined, measured, analysed, improvements implemented and then controlled.
Service Evaluation:
- An internal evaluation of a service provided to a select set of patients for a given period of time in order to identify issues/good practice and implement appropriate changes if necessary.
* The JREC will not review these types of studies ethically but the JREC will issue a letter stating the type of study being conducted
- Registration is made via the Registration Form
Clinical Audit Contact Details: Clinical Audit Manager, Ms Sinead Palmer – Sinead.Palmer@tuh.ie, 01-4142855
Quality Improvement & Service Evaluation:
Contact Details, Quality Improvement Lead, Ms Mary Hickey – Mary.Hickey@tuh.ie, 01-4142854